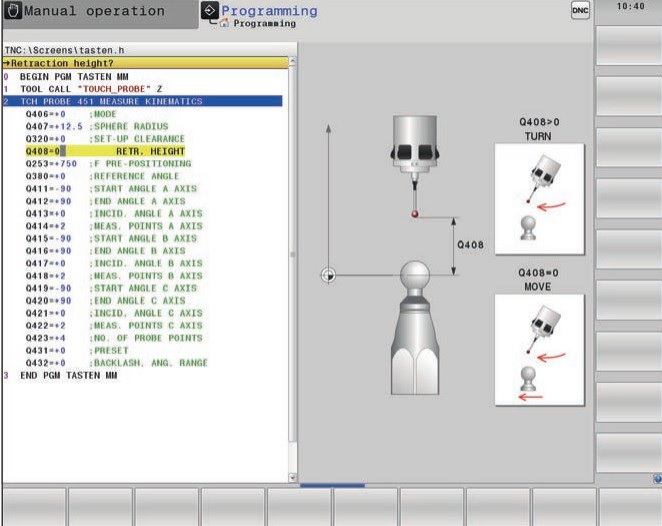
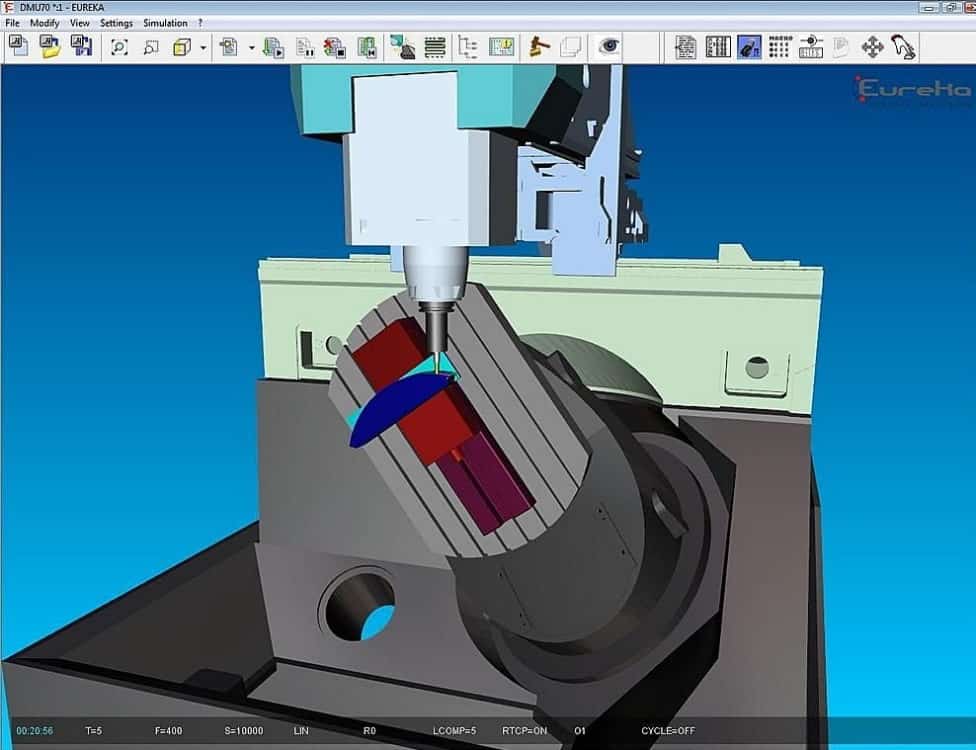
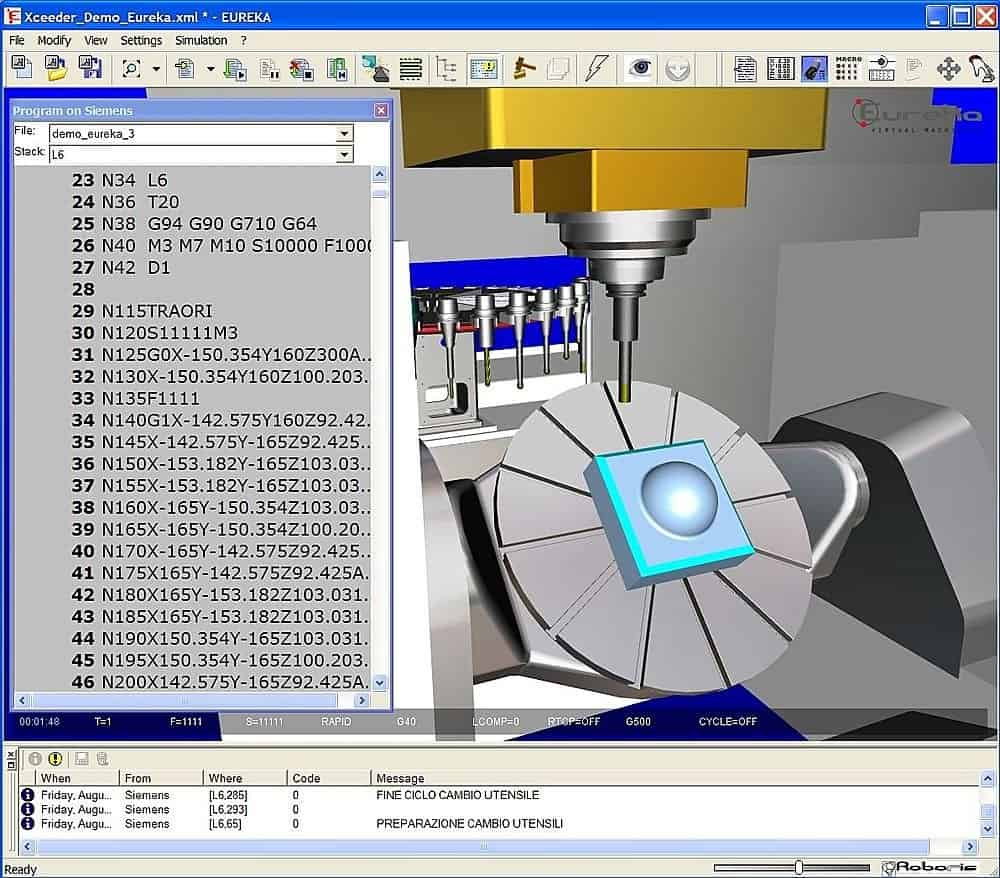
Systemy sterowania CNC firmy Siemens | Marcosta-Obrabiarki do metali CNC konwencjonalne,Znakowarki do metali,Tokarki Uniwersalne,Frezarki CNC,Szlifierki Znakowarki

Sterowanie maszynami CNC. Na czym polega? - Beskidzka24.pl - Regionalny Portal: Bielsko-Biała, Cieszyn, Żywiec, Czechowice, Sucha Beskidzka, Wadowice
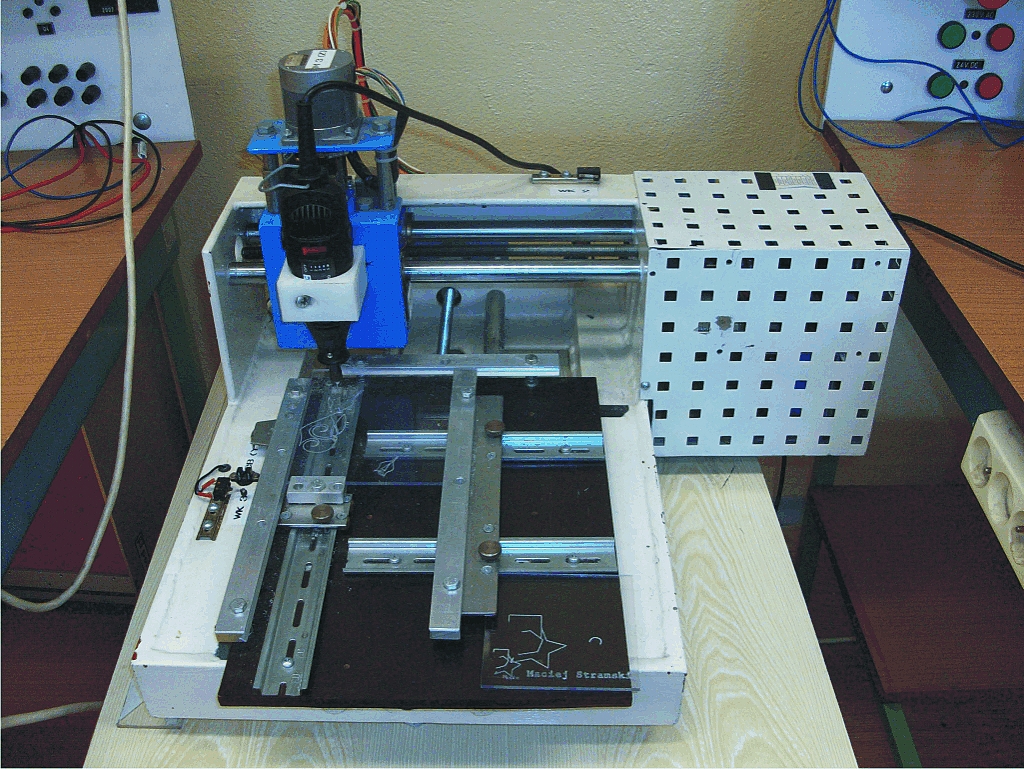
Program sterujący wiertarko-frezarką na podstawie danych z plików DXF / Studenckie Koło Naukowe Spektrum / Forum młodych / Strona główna | PAR Pomiary - Automatyka - Robotyka

Historia G-Kodu | Podstawy CNC | Porady | Maszyny numeryczne | Obróbka skrawania | Technologie obróbki metalu | Mechatronika

Jak jest zbudowany i jakie funkcje ma układ CNC? - OBRABIARKI CNC - SERWONAPĘDY - AKCESORIA CNC - URZĄDZENIA CNC

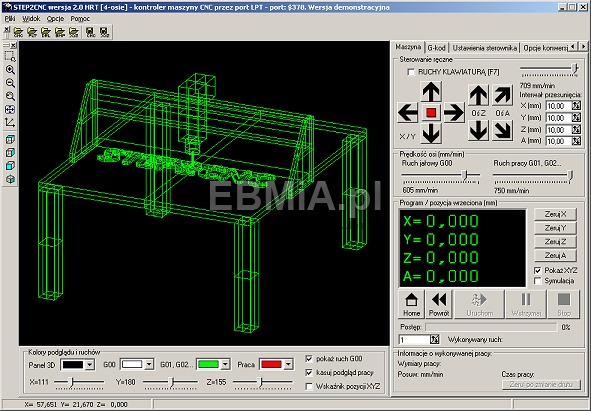
EBMiA.pl - Akcesoria CNC - Program Mach 3 jest najpopularniejszym programem sterującym maszynami CNC 👌 Steruje on pracą silników krokowych, lub serwonapędów wysyłając sygnały kroku i kierunku (Step/Dir) 😉 Przy pomocy programu

Podstawowa konfiguracja programu Mach3 na przykładzie Frezarki 3040Z-DQ ze sterownikiem USB STB4100 | EP.com.pl